News
New paper on electrocatalytically active cobalt oxide spherulites published in Crystal Growth & Design
08th September 2020
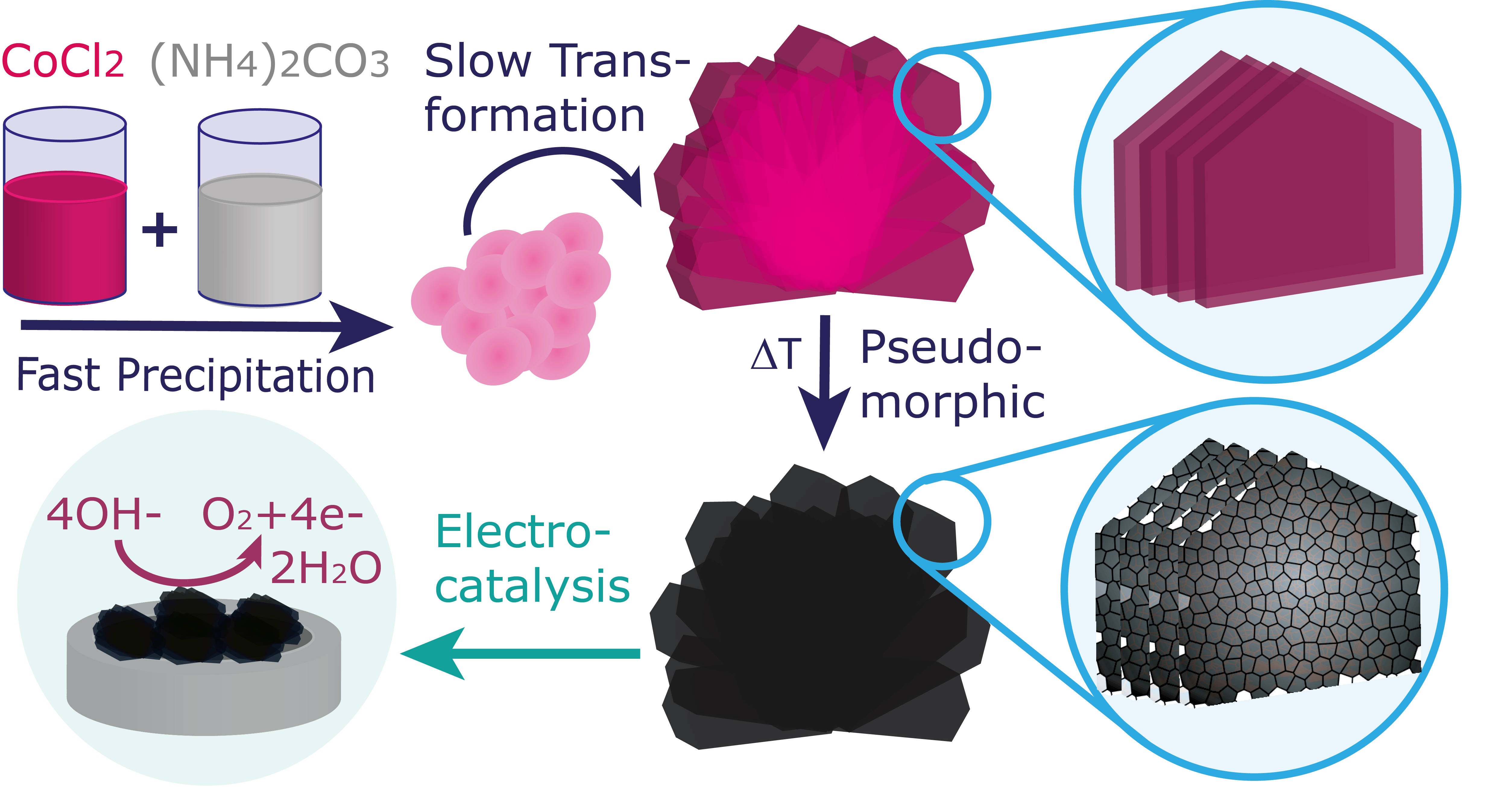
With their tendency to form low-dimensional materials with high surface area, cobalt hydroxide carbonates represent an important class of precursors to Co3O4 – a heterogeneous catalyst with various applications including sustainable energy conversion. We here present a facile methodology for the room temperature precipitation of cobalt hydroxide carbonate under additive-free conditions. Upon ageing in aqueous solution, the initially deposited amorphous bulk solid slowly transforms into macroscopic crystals with an unusual spherulitic morphology interfacically templated by glass-surfaces. Most intriguingly, the individual branches of these hemispherical particles represent highly anisometric, elongated platelets reaching mm-dimensions along the growth direction, while their lamellar architecture indicates the formation of a layered double hydroxide structure. Gradual substitution of Co(II) with Mn(II) results in the deposition of spheroidal stoichiometric carbonates with calcite structure, in which the crystal lattice progressively expands with increasing Mn content. Replacement of Co(II) by Ni(II), in contrast, preserves the spherulite morphology at low Ni(II) content (Co:Ni > 1), but prevents precipitate maturation at higher proportions of Ni(II). Calcination converts the hydroxide carbonate precursor into hierarchical Co3O4 spherulites with superstructures composed of interconnected nanoparticles, where this nanoscale arrangement is shown to positively affect the electrocatalytic activity towards the oxygen evolution reaction.
Read more about this research here.
